Victor C. Baum, MD
- Professor of Anesthesiology and Pediatrics
- Executive Vice-Chair
- Department of Anesthesiology
- Director, Cardiac Anesthesia
- University of Virginia
- Charlottesville, Virginia
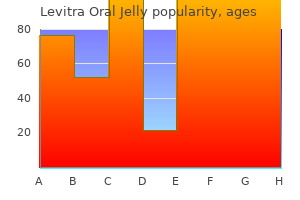
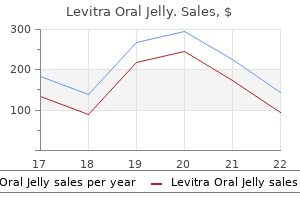
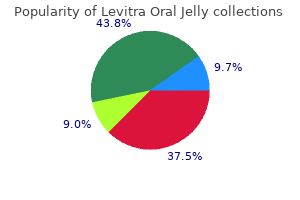
For all trials except 189 for one the methods for treatment allocation concealment were judged to be unclear erectile dysfunction treatment alprostadil buy discount levitra oral jelly 20mg line. This section presents results derived from 21 placebo-controlled trials that compared the efficacy and harms profile of 180-184 erectile dysfunction drugs at walgreens discount 20mg levitra oral jelly otc,189 erectile dysfunction juice buy levitra oral jelly 20 mg lowest price,191-201 erectile dysfunction treatment austin tx discount levitra oral jelly 20 mg otc,203-206 190 vardenafil (any dose) to that of placebo erectile dysfunction 5x5 buy levitra oral jelly visa. One trial that explored a doseresponse effect of vardenafil erectile dysfunction proton pump inhibitors 20mg levitra oral jelly fast delivery, without using a placebo arm, is reviewed in a later section (vardenafil dose 1 versus vardenafil dose 2). Therefore, this 181-184,189,191-201,203-206 section describes harms reported in 20 trials. This outcome was reported in eight trials, where it was shown 182 that the incidence of any adverse events (number of patients with one or more adverse event ), 184,189,191,193,200,203 was higher (either numerically or with statistical significance) in vardenafil groups (regardless of vardenafil dose or dose regimen) than in placebo groups. The proportions of patients with one or more adverse event in vardenafil groups across the trials ranged from 182 189 about 27 percent (10 mg dose) to 74 percent (20 mg dose). The corresponding proportion 200 189 for the placebo groups ranged from about 17 percent to 52 percent. Most commonly, patients in the vardenafil arms experienced headache, flushing, rhinitis, and dyspepsia. Two of the 20 trials did not report the proportion of withdrawals due to adverse events. The withdrawal rate in vardenafil groups across the 18 181-184,189,191-199,201,203-205 193,195,198 192 trials ranged from 0 percent to 5 percent. The corresponding 193,195,203 189 rate for the placebo-treated patients ranged from 0 percent to 6 percent. Some of the reported specific events leading to the withdrawals were myocardial infarction, proctalgia, aortic 212 182,192 181,182 192 bifurcation graft, abnormal liver enzyme levels, myalgia, flushing, nausea, 181,191,192 192 181 headache, kidney calculus, abnormal vision, and rhinitis. The absence or occurrence of serious adverse events could not be 189,199,206 ascertained for three trials. The specific serious adverse events observed across the trials 198 198 in patients after random assignment to vardenafil therapy were: skin ulcer, reflux disease, 197 198,201 198 201 unstable angina, myocardial infarction, syncope and encephalitis aortic bifurcation, 182 facial palsy, and appendicitis. Serious adverse events that occurred in 10 trials were not 181,183,184,191,192,194,196,203-205 193,195,200 specified. In general, judging from the results of these trials, there were no obvious numerical or statistical differences in the occurrence of serious adverse events between patients randomly assigned to receive vardenafil and those assigned to placebo. In 11 trials vardenafil 180-183,189,192-195,198,205 was administered at a fixed dose (5 mg, 10 mg, 20 mg, and/or 40 mg). Ten trials administered 184,191,196,197,199-201,203,204,206 vardenafil with a flexible daily dose (5 mg, 10 mg, 20 mg). There were 10 trials with two or more dose181,183,189,190,192-195,198,205 specific arms of vardenafil. None of the trials were designed to compare flexible and fixed dosage regimens of vardenafil. In one multicenter North American study, for example, after 26 weeks of treatment with 5 mg, 10 mg, or 20 mg of vardenafil or placebo 19, 33, 42 and 7 percent of patients, 192 respectively, experienced at least one adverse event. In a trial of similar design of 12 weeks 189 duration, these proportions were 57, 63, 74, and 52 percent, respectively. The similar trend was observed in a trial that compared 20 mg and 40 mg doses of vardenafil (47. The most frequently observed adverse events in the 10 trials were 190 headache, flushing, dyspepsia, or rhinitis. In one trial, eight and 13 patients developed visual 189 disturbance(s) in the 10 mg and 20 mg groups, respectively. In another trial, two patients (one patient in each 5 mg and 20 mg groups) were observed to have visual disturbances (sensory, abnormal vision, and brightening). In three trials, none of the patients treated with 181,183,189,190,192,194, vardenafil withdrew because of adverse events. For the remaining seven trials, 205 the rate of withdrawals was numerically similar between treatment arms using 10 mg versus 181,183,189,190,192,194,205 20 mg of vardenafil. There was no apparent numerical or statistically significant difference in the occurrence of serious adverse events across the treatment arms of various doses of vardenafil. In another study, the corresponding proportions of patients with at least one serious adverse event were 5, 3, 192 190 and 4 percent. Four deaths were reported during one trial; one death resulted from suicide (10 mg group), while the other three (in the 20 mg group) occurred after myocardial infarction, coronary angioplasty, and ischemic cardiomyopathy. Results from two other trials demonstrated trends of a numerical increase in the rate of improved erections across 5 mg, 10 mg, and 20 mg doses of vardenafil. The highest proportion of patients with improved erections was observed in the 20 189,192 181 mg groups (range 80. In another trial, the proportion of participants with 49 improved erections was higher in participants who received 20 mg compared with those who received 10 mg of vardenafil (72 versus 57 percent, p < 0. Quantitative Synthesis Meta-Analysis of Trials Series of meta-analyses were performed using efficacy and harms data obtained from the 180-184,189,191-201,203-206 reports of 21 trials that were conducted in: 1) Clinically heterogenous groups of patients 2) Clinically homogenous groups of patients Clinically heterogenous groups of patients vardenafil (any dose: 5 mg, 10 mg, 20 mg, 40 mg) versus placebo. The analyses presented in this section did not include 10 trials for the following reasons: distinct clinical groups of patients. Thus, there remained 11 trials that were potentially eligible for meta 182,184,189,191,192, 194,197,198,200,201,203 analysis. One of the 184 trials was restricted to patients who were nonresponders to previous treatment with sildenafil. This difference between the populations of the two trials might have led to the high degree of 2 statistical heterogeneity that was found (I = 61 percent) (Figure 39). The second meta-analysis, 184 which did not incorporate the trial of sildenafil nonresponders (see Figure 42), yielded a 2 substantially lower degree of heterogeneity (I = 3. This meta-analysis incorporated data from 10 182,184,189,191,192,194,197,198,201,203 trials. This meta-analysis incorporated the results of nine 182,191,192,194,197,198,200,203,213 trials. This meta-analysis included six trials, 192,194,197,198 184,189,200,201,203 the outcome of dyspepsia was not ascertainable for five trials. Only three trials including diabetic patients were potentially suitable for meta-analysis. Meta-analyses for efficacy outcomes in diabetes patients were not performed in view of missing qualitative or quantitative information. This meta-analysis included results from three 181,204,205 trials of patients with diabetes. There were 10 trials with two or 181,183,189,190,192-195,198,205 more dose-specific arms of vardenafil. The analysis in this section 181,183,205 193,195 excluded trials of distinct clinical groups of patients and crossover trials. Therefore, 189,190,192,194,198 five potentially eligible trials remained for the analyses. The occurrence of serious adverse events could not be 189,194 ascertained for two trials. Three meta-analyses, each 192,194,198 incorporating results from three trials, were performed separately for the incidence of headache, flushing, and dyspepsia (Figures 57?59). Assessment of Publication Bias Funnel plots were generated and examined to graphically assess the extent of asymmetry. The following list shows the reference identifications for these trials and corresponding publications (each row). Of the two Italian trials, one was funded by Pfizer; the other did not report the funding source. Of the 22 parallel-arm trials, 13 had two arms and 215,221,226,227,229,230,235,237,238 nine trials had three or more arms. Of the 30 trials, 23 were placebo 215-227,229,230,233-240 controlled and seven were active-arm. Further information on trial characteristics is provided in Table F-3 (Appendix F). The total and mean numbers of patients randomly assigned to study interventions or placebo across the 30 trials were 10,718 and 358, 232 respectively. The number of patients randomly assigned across the trials ranged from 20 to 214 4,262. Other 232-235,237-240 234-236,238 exclusion criteria were cancer chemotherapy premature ejaculation, spinal 215,219,233-235,239 103,217,233,235,236,238 cord injury uncontrolled hypertension, use of alpha 163,238 216,221-223,233,234,236 blockers/androgens, and diabetes. One trial additionally excluded patients 233 with prostate-specific antigen levels >10 ng/mL. Three trials 236,237,240 238 included Southeast Asian, one trial Japanese, and one trial Turkish and Egyptian 234 patients. The approximate proportion of Caucasians in the remaining 17 trials ranged from 73 224 163,220 218, percent to 100 percent. The presence or 121,218,219,222,225,233 absence of comorbidities could not be ascertained for six trials. In 103,118, 214,218,220,227-229,232,237-239 232 other 12 trials this proportion ranged from 20 percent to 29 228 215-217,223,224,230,235,236 216,236 235 percent, and in eight trials from 30 percent to 43 percent. The 121,163, 219,221,222,225,226,233 remaining eight trials failed to report the proportion of hypertensive 103,118,214,215,224,229,241 patients. The authors of 13 163,216,222-224,226,230,233,235-237,239,240 trials did not report the proportion of smokers. In seven 118,121,223,228,229,234,236 trials, this proportion was from 20 to 30 percent. Interventions Patients across the 30 trials that were reviewed received oral tadalafil monotherapy in either 215,221,226 experimental or active control arms. In most of the trials, tadalafil was given in 10 mg 230,237,238 118,121,163,214-220,222-230,232,234,236-240 221 and 20 mg doses. One trial included three additional 238 randomized arms in which patients received 2 mg, 5 mg or 25 mg of tadalafil. In another trial, one additional arm of randomly assigned patients received 5 mg of tadalafil. In one placebo235 controlled trial, patients were randomly assigned to receive either 2. In addition to these three trials, a 118,121,163,217-220,225,235 fixed dose of tadalafil was used in nine others. The duration of tadalafil treatment across the trials ranged from about 4?6 214,215,218,230,232,233,239 216 weeks to 24?26 weeks. In half of the trials, tadalafil was administered for 103,118,217,219,220,222-224,226-229,234,236-238 about 12 weeks. Outcomes In total, all 30 trials reported some information on the absence and/or occurrence of either total or serious adverse events. In four trials, the incidence of any adverse events was not 121,217,221,224,232 reported. Authors of 14 trials failed to report the absence or occurrence of serious 118,121,163,216,218,219,221,225-227,229,230,232,237 adverse events. The number of patients who withdrew as a 221,232 result of adverse events was reported in all but two trials. Study Quality and Reporting the mean Jadad total score for the 30 included trials was 3. The individual Jadad total 163 216,222,225 103,163,214, 219,228, 232 score for 30 trials ranged from 1 to 5. Three trials could not have been double blinded because patients received either 214,228,232 on-demand or fixed dosing regimens of tadalafil. Of the 24 double-blind trials, only nine 118,216,218,221,222,224,225,227,239 trials reported some description of the blinding method(s) used. Only 219,238,239 three trials reported some information on the allocation concealment, which was deemed to be adequate. The adequacy of allocation concealment for the remaining 27 trials could not be ascertained. The length of washout period 118 121,228,232 for the seven remaining crossover trials ranged from 4 days to 14 days. The occurrence of total and serious adverse events across the 23 placebo-controlled 215-227,229,230,233-240 trials was reported poorly. For example, in one trial, the proportion of patients who experienced at least one adverse 222 event in the tadalafil and placebo arms were 51. Even though the proportion of patients in one trial was numerically greater in the tadalafil arms (39. Most common adverse events reported across all trials were headache, back pain, dyspepsia, dizziness, nasal congestion, flushing, and myalgia. In general, the occurrence of these events tended to be numerically more frequent in tadalafil arms than in placebo arms. Moreover, a statistically significant higher incidence of these 215,220,222,223,225,226,239 events was reported across several trials in tadalafil versus placebo arms. The majority of the trials reported that tadalafil was well tolerated and that patients had had adverse events mostly of mild or moderate severity. Eleven of the 23 trials did not report whether there had been any occurrence of serious 216,218,219,221,225-227,229,230,237,239 adverse events. Of the 12 trials that reported any occurrence of 215,220,222 serious adverse events, three trials did not specify what these events were. The proportion of patients who withdrew due to adverse events across trials was five?six 217,222,224 215-220,222-227, 229,230,233-240 percent or less and similar across the tadalafil and placebo arms. In general, the results of the 23 placebo-controlled trials showed that patients who received tadalafil (10 or 20 mg) experienced greater improvement in erectile functioning. The corresponding mean treatment 216 237 response change in placebo arms ranged from 0. Furthermore, results of two trials indicated that patients receiving even lower doses of tadalafil (2.
But when for various reasons erectile dysfunction and stress buy levitra oral jelly overnight delivery, the body can?t properly regulate glutamate erectile dysfunction treatment manila discount levitra oral jelly 20 mg with amex, as often occurs in the children I see erectile dysfunction at 55 trusted 20mg levitra oral jelly, glutamate can build up to toxic levels erectile dysfunction zocor order levitra oral jelly without prescription. Tese ingredients are added to food by food scientists working for major processed food producers impotence early 30s 20 mg levitra oral jelly otc. The companies add these chemicals to processed foods because excitotoxins fool the brain into experiencing a particular food as tasty impotence drugs buy discount levitra oral jelly. Unfortunately, excitotoxins also raise levels of excitatory neurotransmitters, including glutamate, potentially causing nerve-cell death. This can result in an infammatory reaction while also depleting other intermediates in the body. Studies indicate that glutamate can increase blood pressure but may also cause irregularities in blood pressure. Although in my experience, most of the children beneft from lowering glutamate levels, you and your practitioner can make an individual determination for your child through lab tests or monitoring symptoms. They function a bit like fshing poles and each type has a specifc task?to reel into the cell a specifc neurochemical. However, these receptors can also pull in other excitatory neurotransmitters which thereby enter the cell. The following neurotransmitters are pulled into the cell via glutamate receptors: Homocysteine More glutamate receptors will tend to pull in more excitatory neurotransmitters, causing higher levels of excitatory chemicals within each cell. In certain key studies, scientists found that individuals with autism had elevated excitatory neurotransmitter levels compared with non-autistic individuals. I suspect that people with autism may produce more receptors for glutamate and other related excitatory chemicals. The combination of glutamate and excessive calcium makes it impossible for the neurons (nerve cells) to rest. The neurons continue to fire without stopping, causing the release of inflammatory mediators, which trigger additional calcium influx. This ongoing firing process results in neural cell inflammation and the death of neurons. Mark Neveu, a former president of the National Foundation of Alternative Medicine, said when speaking of excitotoxin damage, glutamate is the gun and calcium is the bullet. This is the main reason that, on this program, I recommend caution in supplementing with calcium. Both limiting calcium intake and supplementing with magnesium and zinc can help maintain low to moderate calcium levels. This strikes a balance between maintaining necessary amounts for bone health without damaging the nerves. As you can see, achieving the appropriate levels of key minerals is also foundational. This will help you precisely determine current levels as well as monitor your success following supplementation. Autism: Pathways to Recovery 89 With these basic guidelines, and after checking with your doctor, you can use the following supplements to help achieve the proper levels of key minerals. Glutamate and Intelligence Children with autistic type behavior have often been described as exceedingly intelligent, with a certain percentage considered to be savants, people with extraordinary mental ability in certain areas (such as an exceptional memory). Z Tsien and his collaborators have demonstrated a correlation between glutamate receptors and superior ability in learning and memory. However, his research indicated a signifcant downside: enhanced levels of glutamate were also correlated to an increased risk of stroke and seizure activity. The takeaway from this study is that more highly intelligent people often have higher glutamate receptor activity. At the same time, higher receptor activity may make intelligent people more vulnerable to glutamate excess and nerve damage. In my view, lowering glutamate levels to prevent nerve damage is a sound preventive measure for nearly everyone. To restore balance, we frst must decrease glutamate levels by limiting foods that contain glutamate. Step One, Part One Other Factors That Tip the Balance Controlling glutamate intake is vital, but other factors also play a part. This may explain why children with autism who have received these shots may be more vulnerable to this imbalance. If so, then addressing chronic viral infections as we do on this program could potentially help. Nutrigenomics can also tell us which individuals can most beneft from B6 supplementation and help determine when to add B6. This is just one example of how Nutrigenomic information helps to introduce the right support in the right time sequence. Lowered ability to remove glutamate Glutamate stimulates insulin release, which in turn lowers glucose. Terefore, a drop in blood glucose disrupts this removal process and allows the build up of toxic glutamate. In fact, hypoglycemia, or low calorie/ starvation conditions induce the release of glutamate and reduce the ability to remove excess levels of glutamate from the brain, making it essential to provide regular nourishment through meals and snacks to maintain consistent blood sugar levels throughout the day. If after getting your test results, you learn that you or your child have that mutation, you may want to revisit the recommended pancreatic supports and increase them if needed. Glutamate prompts the body to release opioids to protect the brain from damage, contributing to the spaciness that some of the children experience. Elevated glutamate levels can also deplete glutathione, a major antioxidant that promotes healthy detoxifcation and prevents infammation. With lowered glutathione, there will be an increased tendency toward leaky gut (or Irritable Bowel Syndrome). Since glutathione helps to protect neurons from damage, its depletion also results in nerve cell death. Glutamate excess can also contribute to insomnia, bedwetting, and problems with eye focus and making eye contact. In fact, often when they frst recover language, their frst communications will sound like a run-on sentence. If you don?t yet have your results, please use the supplements recommended for everyone. Don?t be surprised if they show up in foods you have served your children all along! Later in this chapter, you will fnd lists of ingredients to be avoided, as well as lists of the types of products in which these harmful ingredients are most commonly found. According to the manufacturer, Varivax-Merck chicken pox vaccine (Varicella Virus Live) contains L-monosodium glutamate and hydrolyzed gelatin, both of which contain processed free glutamic acid. Both glutamate and aspartate occur naturally in many foods, such as proteinrich foods, wheat gluten, hydrolyzed yeast, and milk casein. You can see why a grilled-cheese sandwich should probably not be on the menu of most children with autism. Many parents encourage their children to drink smoothies, but beware of the protein powder you use. While the amino acids that make up protein are necessary for normal brain function, most protein rich foods contain high levels of glutamate and aspartate which is why I don?t recommend high protein diets for this population. Further, high-protein diets force the body into a state of cannibalism called metabolic acidosis, in which blood levels become so acidic that the body starts feeding on muscle tissue for nutrients. Many children with autism have elevated ammonia levels, yet another reason for them to avoid high-protein diets. Many nutritional supplements contain glutamine (or glutamic acid), because it helps restore gut health and integrity. However, glutamine is readily converted 98 Autism: Pathways to Recovery Chapter 4. Step One, Part One to glutamate, so I counsel parents to read supplement labels and avoid products containing glutamine. However, it is necessary to avoid excessive intake in order to stop the infammatory process that glutamate and other excitotoxins trigger. To help our nerves function, we need the stimulatory activity provided by the glutamate receptor. But you can regard these foods and ingredients with suspicion, as they have been known to cause problems. Even if you do not eliminate them entirely, once you are aware of these concerns you can pay special attention after you or your child have eaten a potentially troublesome food and notice if there is an immediate negative reaction (within the next twenty-four hours. More often, there is a long term cumulative efect, making it harder to trace the various factors contributing to an illness or symptom. To understand how this works, frst visualize a measuring cup as a metaphor for what is going on within us that we cannot see. Like a human body, the overfowing cup can no longer contain or manage the accumulation of toxins to which it has been exposed. The bottom line is that once glutamate reaches a certain level, neurological damage has already occurred. To avoid getting to that point, the goal is to limit the amount of glutamate that comes into the body every day. As you learn the sources of glutamate and aspartate, you can make informed choices about limiting intake. The aim is to keep excitotoxins to a minimum; you will never avoid them completely. Transitioning to proper nutrition and getting glutamate under control are foundational to this program, but I don?t underestimate the challenge. Please seek out the techniques and approaches that work for your child and family, and remember that this is a marathon, not a sprint. Promoting Healthy Digestion fter you receive the Nutrigenomic test results, (in Step Two), you will put into place the supports for methylation cycle function. With those A supports, you will be naturally undergoing detoxifcation as well as addressing microbial imbalances more intensively. The digestive supports that I?ll suggest in this chapter can be continued throughout the entire program. As we repair and regenerate the digestive organs, the body is better able to receive nutrients and release toxins which are critical for Step Two of the program. Moreover, strengthening the digestive tract creates a better environment for addressing microbial overgrowths. In Step Two, we will upgrade our eforts to eliminate inhospitable bacteria and viruses. The organs that require support may include the liver, kidneys, pancreas, stomach, and intestinal tract. In addition, we will also address key hormones and neurotransmitters that lay a groundwork for wellbeing. For example, if a child is constipated, or has just undergone a course of antibiotics, many parents already understand that it would be good to restore healthy gut bacteria through probiotics. If a child has many allergies, both gut support and immune building supplements would be obvious choices. In addition, when tests reveal the presence of bacteria, yeast, or other microorganisms, following the protocols to support gut health is advisable. However, a child may need additional support without that being immediately obvious. Terefore, in this chapter, I will list supplements for specifc purposes as well as the test results (and symptoms) that might indicate a need to take them. I?m not recommending that you test your child in every instance, as for many people that is too costly. However, if you see symptoms that you or your practitioner believe could indicate the need for specifc organ support, you can always confrm your supposition by testing. Autism: Pathways to Recovery 103 In addition, there are certain tests that are good to take as a baseline before you bring in methylation supports at Step Two. Tese can either be undertaken at the beginning of Step One, during Step One, or at the very beginning of Step Two. If you run this test through my ofce, It will be held until your Nutrigenomic test results arrive, and then it will be used to fne-tune your recommendations. You can also retest to fnd out if supplementation is producing the results you seek. Although I won?t touch on the wide range of interactions, certain ones are critical in addressing detoxifcation. The Liver The liver is one of the most important organs of the body, working to metabolize carbohydrates, fats, and proteins. It stores vitamins and minerals and contains regulatory mechanisms for healthy blood sugar and hormonal levels. In addition, infection, infammation, and low levels of the mineral, magnesium can also lower glutathione. Fortunately, certain herbs and other supplements are very helpful for maintaining liver health. Further, addressing mutations in Step Two should also help the body to create glutathione. You can elect to use the supports suggested, as well as take certain optional lab tests listed below. Chronic viral infections and detox both stress the kidneys, creating the need for kidney support. Also, if there are any indications of chronic viral issues, such as elevated antibody titers, I would also recommend a consistent form of kidney support. Organs function in tandem, so it is important to look at the optimal function of each of these digestive organs.
The involvement of activated complement and targeted macrophage destruction was visualised erectile dysfunction beat purchase discount levitra oral jelly line, and antibodies in the serum of patients to gangliosides and ganglioside like epitopes resulting in immunological molecular mimicry were described impotence icd 9 code discount levitra oral jelly 20 mg without a prescription. This paper re-ignited the quest for models which had been fallow for some years and re-engaged the anti-ganglioside antibody hypothesis erectile dysfunction vacuum pump price buy levitra oral jelly 20mg mastercard. Darwin erectile dysfunction unani medicine purchase levitra oral jelly 20mg visa, in his paper on the Formation of Vegetable Mould erectile dysfunction treatment tablets levitra oral jelly 20 mg amex, had actually demonstrated that worms were very sensitive to the vibrations of the piano when they were placed on the sounding board and middle C (256Hz) was sounded impotence 17 year old male purchase levitra oral jelly 20 mg. So over 170 years we have discovered that worms can?t hear but they can feel middle C! My key paper for this decade is not yet published but derives from the work of Hugh Willison with complement inhibition. Halstead and colleagues demonstrated the complete abrogation of an antibody-mediated acute inflammatory neuropathy in mice [16]. We should be proud of our discoveries, sometimes serendipitous, sometimes deliberate. We should continue to pursue our goal to cure this disease?we have not done so badly thus far. Guillain G, Kreis B (1937) Sur deux cas de polyradiculo-nevrite avec hyperalbuminose du liquide cephalorachidien sans reaction cellulaire. Guillain-Barre Syndrome Study Group (1985) Plasmapheresis and acute Guillain-Barre syndrome. We apologise if there are even more worthy forgotten papers that we have overlooked, especially gems hidden within other languages. Increased intracranial pressure caused by increased protein content in the cerebrospinal fluid; an explanation of papilledema in certain cases of small intracranial and intraspinal tumors, and in the Guillain-Barre syndrome. Brain-stem encephalitis; further observations on a grave syndrome with benign prognosis. This is intellectually the easiest consideration because it accepts the hypothesis. There is some limited support for this idea of subgroup analysis in the context of the 1976?1977 influenza vaccine, or for certain infections [18,19,20]. The cross-reactive response may be the norm, but only certain individuals open the blood nerve barrier, permitting the development of a clinical rather than just an immunological process. Natural killer cells are an integral part of the cellular innate immune system, with important links to the adaptive immune system. Initially such cases were not accepted as being a disorder of the peripheral nerves, as anterior horn cell changes were present, suggesting this was spinal in aetiology. Slowly this evolved into acceptance that the peripheral nerves were involved, and the anterior horn cell changes were a postmortem artefact. Later studies reviewed indicated that pathological changes were patchy and particularly where anterior and posterior roots join to form the spinal nerves. These demonstrated that the time from onset to death is an important contributor to the pathological changes present. Earliest is the presence of oedema and irregularity of the myelin sheaths, with cellular infiltration only 9?11 days after onset. Sur un syndrome de radiculo-nevrite avec hyperalbuminose du liquide cephalo-rachidien sans reaction cellulaire: remarques sur les caracteres cliniques et graphiques des reflexes tendineux. It may seem strange to end a chapter on things forgotten with a paper that started the book! When authors newly describe a syndrome, a distinction is being made from what is already known. So when they wrote the striking hyperalbuminosis of the cerebrospinal fluid without cellular reaction is a feature of signal importance it has not been described in radiculitis and polyneuritis [38], what were those other cases of sometimes febrile radiculitis and polyneuritis with a cellular reaction? These were doctors working in an army hospital in the Great War, seeing soldiers from the trenches, in northern France. In addition to the war wounds there were numerous infections, arising from poor hygiene, as well as zoonoses, with endemic rats and lice in the fields and forests behind the front, and Ixodes ricinus ticks, the European vector for Lyme neuroborreliosis, even now prevalent in this part of France [39]. Febrile polyneuritis was discussed contemporaneously, by Lieutenant-Colonel Gordon Holmes, Consultant Neurologist, British Armies in France [40]. Richard Hughes writes, It is puzzling to know what modern disease Osler and Holmes were describing since absence of fever is the rule in GuillainBarre Syndrome [41]. However, Lyme disease and trench fever may be forgotten differentials for the historical context. It is still the case that a lumbar puncture is usually performed to rule out infectious diseases, such as Lyme disease [43]. Trench fever, a systemic infection of Bartonella quintana transmitted by the body louse, was rife and may cause meningoencephalitis with cellular pleocytosis. Trench fever caused marked loss of fighting man power and consequent investigation [44]: It is curious that the sniffing up the nose of the infected excreta of lice did not give rise to infection, whereas the placing of the same material in the conjunctival sac did give rise to positive results in 2 cases. Amit R, Shapira Y, Blank A, Aker M (1986) Acute, severe, central and peripheral nervous system combined demyelination. Yuki N (2009) Fisher syndrome and Bickerstaff brainstem encephalitis (Fisher-Bickerstaff syndrome). Yoshii F, Shinohara Y (1998) Natural killer cells in patients with Guillain-Barre syndrome. Said G, Hontebeyrie-Joskowicz M (1992) Nerve lesions induced by macrophage activation. Stoll G, Jung S, Jander S, van der Meide P, Hartung H P (1993) Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. Vandenesch A, Turbelin C, Couturier E, Arena C, Jaulhac B, Ferquel E, Choumet V, Saugeon C, Coffinieres E, Blanchon T, Vaillant V, Hanslik T (2014) Incidence and hospitalisation rates of Lyme borreliosis, France, 2004 to 2012. Bruce D (1921) Trench fever: final report of the war office trench fever investigation committee. However, the epidemiology of the syndrome was poorly understood; epidemiology as a distinct medical discipline was still in its early stages at this time, and the relative rarity of the condition made epidemiologic studies challenging. Information on incidence, demographics, and other basic epidemiologic data were gleaned from larger studies on the occurrence of neurologic disease in general. So many different systems of classification and characterization of the syndrome began to evolve that it eventually prompted Guillain himself to state, I no longer recognize the syndrome J. The case definition used was a clinical history of acute or subacute onset of bilateral weakness with or without cranial nerve abnormalities or sensory findings, in the absence of concurrent febrile illness; cyto-albuminologic dissociation was assessed for but was not a requisite. During that 34-year period, 29 patients meeting the case criteria were identified, resulting in a mean annual incidence of 1. Rates were highest in the 40?59 age group (though, notably, due to small sample size the standard errors in each age group were large). An antecedent respiratory or infectious illness closely preceding neurologic illness onset was reported in 16 (55%) cases. The Lesser study was followed up by a subsequent assessment using the same methodology and the same database, extending the investigation period through 1976, and included a case-control component [3]. The additional 8 years yielded 11 additional cases, with a total of 40 cases identified between 1935 and 1976. The findings of this assessment were largely the same as the first assessment: overall mean annual incidence of 1. Even the authors of these studies cautioned against generalizing these findings to the entire United States, let alone the world. Globally, what work was being conducted consisted of case reports and case series using varying classification schemes, case ascertainment methodologies, and denominators resulting in a vertigo-inducing variety of estimates of incidence, seasonality versus no seasonality, age distributions and other basic epidemiologic parameters. Essentially, the only consistent feature of these various estimates and assessments was inconsistency. After this laborious process, a resultant 63 papers survived to the point of full review. These differences resulted in a vast range of incidence estimates with incidence rates varying between 0. Invariably, incidence estimates provided by prospective studies and database searches were higher than those found by retrospective studies relying on medical record review. To obtain the most accurate incidence estimates, we applied specific and tight criteria. This study identified 1,683 nonduplicative publications, of which 16 met the inclusion criteria. The authors identified 36 patients in Harbin, resulting in a crude incidence of 0. The 2 notable findings from this study were the relatively low crude incidence when compared to other studies using such robust case-finding methodologies, and the finding of a high incidence among children and lower incidence in adults. This study assessed incidence only in children <15 years, precluding a comparison of incidence between children and older age groups as in the Harbin paper. It is possible to find case reports or case series of the development of nearly any neurologic illness following nearly any vaccine; reports of X illness following Y vaccine permeate the literature. However, substantiation of an etiologic or causal nature of such associations with data from clinical trials or large epidemiologic studies is generally lacking. Thus, the occurrence of many clinical events that are associated with a particular vaccine by virtue of temporal proximity is substantially different than demonstrating a causal relationship. These isolates were antigenically similar to the virus responsible for the catastrophic 1918 swine flu pandemic that resulted in millions of deaths worldwide. Surveillance around the Fort Dix area failed to identify the presence of the H1N1 virus outside of the base; surveillance among the Fort Dix military personnel, however, demonstrated sustained person-to-person transmission. In March 1976, a panel of experts was emergently convened, and recommended widespread H1N1 vaccination in anticipation of another epidemic of swine flu. Keep in mind, to this point, the H1N1 virus had not been identified off of the Fort Dix base, and no one had died or fallen severely ill from the virus. Over the subsequent 11 weeks, about 45 million persons were administered the vaccine. Before the campaign was launched, a nationwide passive surveillance system was established to evaluate any possible adverse events following this immunization. On the basis of these preliminary findings, and the weakening evidence that a swine flu pandemic was actually going to emerge, the vaccination campaign was suspended on 16 December 1976 (Dr Schonberger recalls the secretary of Health and Human Services telling him in a stern and somewhat irritated voice, You better be right about this. The distribution of cases occurring by week after vaccination clustered in the first 5 weeks, particularly in weeks 2 and 3 after vaccination. During the 6-week period after vaccination, the attack rates in each of the 4 adult age groups, (18?24, 25?44, 45?64 and 65+ years) but not for children (0?17 years), were significantly elevated compared to the background rates. Dr Schonberger published his final results in the American Journal of Epidemiology [10]. These points were summarized in a scathing editorial published in the Archives of Neurology by Kurland and colleagues [11]. In determining risk, the panel decided after analysing available data to exclude many cases that were included in the original study, and to base its risk assessment on the most definite and severely affected cases (those with extensive motor involvement?). The panel reported that this vaccine effect possibly lasted for 8?10 weeks, but not longer. Perhaps the most creative attempt to establish a biological underpinning to this association was performed by Nachamkin and colleagues [14]. This study assessed the potential association of influenza vaccines with anti-ganglioside antibodies. Additional exploration of the role of vaccine proteins, including use of negative controls of a type not utilized in the study, would be needed to further substantiate these findings. Of these 9 studies, 2 involved active, population-based surveillance, medical record reviews and patient interviews [19,24]. One used hospital discharge data without a link to vaccination information [18] and one assessed reports to a national passive vaccine-adverse event surveillance system [21]. Unfortunately, unlike the 1976 H1N1 influenza virus, the 2009 virus was definitely a serious public health threat; in April 2009, the virus was identified in specimens obtained from 2 epidemiologically unlinked patients in the United States. The global emergence of the pandemic (H1N1) 2009 virus (pH1N1), and its rapid global spread associated with community-wide outbreaks, hospitalizations and deaths prompted rapid development of new influenza A (H1N1) 2009 monovalent vaccine that could be produced in sufficient quantities to be used globally. Although the safety and efficacy of the influenza A (H1N1) 2009 monovalent vaccine was to be assessed through a small number of limited clinical trials, the interval between vaccine development/manufacturing and widespread use of the vaccine was extremely short, pre-licensure safety data was quite limited, and post-licensure safety surveillance was going to take many months to collect and assess. Surveillance commenced on 1 October 2009 (coincident with the introduction of the U. Trained surveillance officers reviewed medical records and conducted telephone interviews with suspected cases to obtain basic demographics, risk factors and vaccination status, and determined date of receipt of p(H1N1) and seasonal influenza vaccines. Antecedent events were less common among cases who received p(H1N1) vaccine in the 42 days prior to onset compared with those who did not (59% vs. Examination of specific antecedent event types showed that upper respiratory or influenza-like symptoms were the only category that was significantly less common among cases receiving p(H1N1) vaccine than those who did not (38% vs. Subsequent seasonal influenza vaccines, including the 2009 p(H1N1) swine influenza vaccine, have not demonstrated such increased risk, with rare exceptions. These temporal associations are made even more challenging by the fact that it is nearly impossible to exclude the possibility that another antigenic stimulus. This was presumed to be due to the presence of brain protein in the formulated vaccine, with the possible generation of antibodies cross-reactive to peripheral nerve proteins. The vaccine was licenced in January 2005 for use in the United States for persons aged 11?55; in February 2005 a U. Countless millions of lives have been saved by development and implementation of vaccines, and in many ways, vaccines have become a victim of their own?people are concerned about potential complications of vaccines largely because they do not recall the tremendous morbidity and mortality from the very illnesses that vaccines prevent. Stowe J, Andrews N, Wise L, Miller E (2009) Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Poeppl W, Hell M, Herkner H, Stoiser B, Fritsche G, Schurz-Bamieh N, Poeppl G, Gattringer R, Jones N, Maass M, et al. Kinnunen E, Junttila O, Haukka J, Hovi T (1998) Nationwide oral poliovirus vaccination campaign and the incidence of Guillain-Barre Syndrome.
Levitra oral jelly 20 mg amex. Homeopathic medicine for Premature Ejaculation! Erectile dysfunction ! Spermatorrhoea & Nightfall??.