William T. O'Brien Sr., DO
- Clinical Instructor in Radiology
- University of Cincinnati
- Cincinnati, Ohio
N-Methyl nicotinamide does not have biological activity and is a polar fungus in mouth buy 400mg diflucan with visa, water-soluble excretory product fungi definition pronunciation purchase 400mg diflucan with amex. It may be further oxidised in the 6 position of the pyridine ring to give N1-methyl-6-pyridone-3-carboxamide fungus zybez buy 200 mg diflucan with mastercard. High doses of nicotinic acid are excreted in the urine antifungal questions generic diflucan 400mg, as nicotinic acid and its glycine conjugate (nicotinuric acid) (Figge et al fungus fest purchase diflucan line, 1988; Stern et al antifungal cream side effects buy diflucan 150 mg cheap, 1992). Because of the metabolic formation of niacin from tryptophan, the dietary requirements for niacin are complex and related to the dietary content of both tryptophan and niacin (neglecting niacin in cereals, which is largely not bioavailable). The data also show that food supplements (that contain nicotinamide) represent a minor source of intake, even at the 97. Data from studies in animals There is a very limited animal toxicity database on either nicotinic acid or nicotinamide, with the majority of the available studies published before 1960. Weight loss, convulsions and death were reported in dogs given nicotinic acid at 2 g per day for 20 days, but not in dogs treated with up to 1 g per day for 8 weeks (Chen et al, 1938), or in dogs given sodium nicotinate at a dose of 1 g per kg body weight for 63 days (Unna, 1939). Toxicity was not found in rats given 1 g per day of sodium nicotinate in the diet for 40 days (Unna, 1939), whereas nicotinamide inhibited the growth of rats when given at 1% in the diet for 28 days (Handler and Dann, 1942). Suppression of growth in rats was reported when nicotinamide was incorporated into the drinking water to give an intake of 0. A study has reported that nicotinamide was not carcinogenic when mice were given a 1% nicotinamide solution as drinking water for their life-span (Toth, 1983). Niacin deficiency results in birth defects (Chamberlain and Nelson, 1963) and impaired viability; nicotinamide is transferred actively across the placenta (Hill and Longo, 1980; Kaminetzky et al, 1974) and into breast milk (Deodhar et al, 1964). However there are only limited data on the effects of excessive niacin either in utero or during neonatal development. Abnormal neural tube closure defects and other abnormalities were reported when chick egg white was replaced with a solution containing 20 mg of nicotinic acid (Hansborough, 1974) but such experiments are not of value for hazard identification. The limited data available from animal studies did not indicate that either nicotinamide or nicotinic acid was teratogenic, but these were observations from old studies (cited in Schardein, 2000) that would not be considered adequate for risk assessment purposes. Data from studies in humans the principal identification of hazards associated with excessive intakes of niacin have arisen from studies in which high doses of nicotinic acid have been used for its therapeutic effects in lowering blood cholesterol and blood hyperlipidaemias. The most comprehensive study was that conducted by the Coronary Drug Project Research Group (1975). A number of hazards have been reported to be associated with high doses of nicotinic acid. In addition, nicotinamide has been investigated as a method for reducing the risk of the development of diabetes (Knip et al, 2000). Studies have shown that nicotinamide can afford protection in an animal model of immune mediated insulin-dependent diabetes (Reddy et al, 1990), and it has been investigated in a number of clinical trials, some of which are still ongoing. Vasodilatory effects (flushing) Vasodilation is commonly seen in patients given high doses of nicotinic acid for the treatment of hyperlipidaemias. Very large single doses cause hypotension, although tolerance develops to this effect after several days of continued high dose intake. In general, flushing is a mild and transient effect although in many clinical trials it has resulted in patients withdrawing from treatment. The flushing activity appears to be related to the presence of a carboxyl group on the pyridine nucleus since compounds lacking this function, including nicotinamide, are not associated with facial flushing (Bean and Spies, 1940). Flushing is associated with periods of rapid rises in blood concentrations, and sustained-release formulations were developed for the use of nicotinic acid in the treatment of hypercholesterolaemia, in order to minimise this side-effect. Tolerance develops due to decreased formation of prostaglandin D2 on repeated dosage (Stern et al, 1991). Although flushing is not a clearly adverse effect and single oral doses of 100 mg do not alter heart rate or blood pressure, some patients in the study of Spies et al (1938) reported dizziness after oral nicotinic acid (doses not defined). Theoretically if flushing occurred in the elderly, it could exacerbate mild postural hypotension, and could increase the risk of falls, which are a common cause of morbidity in the elderly. This risk relates to taking supplements containing nicotinic acid (not nicotinamide), especially if taken on an empty stomach. Gastrointestinal effects Gastrointestinal effects such as dyspepsia, diarrhoea and constipation are common in patients with hypercholesterolaemia given high doses of nicotinic acid (3 g/day especially as the sustained-release formulation; Knopp et al, 1985). Hepatotoxicity Severe and potentially life-threatening hepatotoxicity has been associated with treatment of patients with 3-9 g nicotinic acid per day for periods of months or years for the treatment of hypercholesterolaemia. Severe cases show liver dysfunction and fulminant hepatitis and may even proceed to encephalopathy requiring liver transplantation. Many of the patients showing hepatotoxicity were taking the slow release formulation of the compound, so that in contrast to the flushing discussed above, the development of hepatic toxicity is a function of long-term chronic exposure to relatively constant levels rather than the fluctuating levels and rapid rises which produce flushing. Glucose intolerance Nicotinic acid (3 g/day) has been reported to impair glucose tolerance in otherwise healthy individuals treated for hypercholesterolaemia. Other effects There have been rare reported cases of a range of effects including blurred vision, macular oedema and increased plasma homocysteine concentrations in patients given high doses of nicotinic acid. These effects were reported at doses similar to those producing hepatic dysfunction, and were reversible upon cessation of high dose treatment. There has been a single report of a possible association with congenital malformation in women taking nicotinamide during early pregnancy (Nelson and Forfar, 1971). On the basis of their retrospective survey of drug and vitamin prescriptions during pregnancy in 1369 mothers, the authors reported that a significantly (P<0. In contrast no such relationship was found during later phases of pregnancy or over the whole of the pregnancy. In that study 1817 women with high risk for producing a baby with neural tube defect were randomised into 4 groups; one group received folic acid, one group a multivitamin preparation (that did not include folate but contained 15 mg/day of nicotinamide), one group was given both preparations and one group received neither preparation. Although the study focussed on neural tube defects, any foetal malformation was recorded together with other pregnancy outcomes, and there was no difference in incidence between the multivitamin preparation and placebo. Vasodilatory effects (flushing) Low doses of nicotinic acid may produce mild but noticeable flushing when taken on an empty stomach (Hathcock, 1997) and this represents the adverse effect detected at the lowest doses. An early study (Smith et al, 1937) reported that a single oral dose of 60 mg nicotinic acid produced marked flushing, which was not associated with changes in heart rate or blood pressure. Spies et al (1938) reported flushing in 5% and about 50% of subjects given single oral doses of 50 mg and 100 mg nicotinic acid, respectively. The dose-response for flushing was examined further by Sebrell and Butler (1938) who gave 3 groups of 6 subjects daily dose of 10, 30 or 50 mg nicotinic acid for 92 days as single oral doses given in solution added to tomato juice and consumed with the mid-day meal; flushing was reported intermittently by 4, 2 and 0 of the subjects given 50, 30 and 10 mg, respectively. The response is possibly related to periods of rapid increase in plasma concentrations of nicotinic acid, because the response is greater after intravenous dosage and is blunted if taken orally with food (Bean and Spies, 1940). The facial flushing associated with low doses of nicotinic acid can be prevented by co-administration of an inhibitor of prostaglandin synthesis such as aspirin (although this is not always recommended Schuna, 1997). Gastrointestinal effects Gastrointestinal effects such as dyspepsia, diarrhoea and constipation are common in patients given high doses of nicotinic acid for hypercholesterolaemia. Spies et al (1938) reported nausea and vomiting in subjects given oral doses of 300-1500 mg of nicotinic acid. Nausea is a common adverse effect in the studies of patients given 3 g of nicotinic acid daily for hypercholesterolaemia. Hepatotoxicity the first report of hepatotoxicity associated with the administration of nicotinic acid was in a study in dogs (Chen et al, 1938), which compared the toxicity of nicotine with nicotinic acid. In that study 2 dogs were given either 145 or 133 mg/kg bw/day nicotinic acid orally and both developed convulsions and excreted blood in their faeces about 2-3 weeks after treatment started. The first case-report of hepatotoxicity of nicotinic acid in humans was in a 23 year old man who developed jaundice after taking 3 g per day for 72 weeks (Rivin, 1959). Subsequent case-reports included a man who had taken 3 g per day for 6 months (Pardue, 1961), and a woman who developed pruritus and jaundice after taking 3 g nicotinic acid (together with 3 g vitamin C and 100 mg pyridoxine for a psychological disturbance) per day for 2. A survey of 66 patients treated with nicotinic acid, of whom 51 had taken 3 g/day for 12 months or more, found a high incidence of abnormal liver function tests (23 patients) while on treatment, with 2 patients developing jaundice (Berge et al, 1961). There have been a number of reports of individual cases of patients with severe hepatotoxicity resulting from the use of nicotinic acid for hypercholesterolaemia or hypertriglyceridaemia. In all cases, cessation of nicotinic acid administration resulted in resolution of the liver symptomatology. A single case report gave some insight into the dose-response relationship for sustained-release nicotinic acid since symptoms of anorexia, fatigue and persistent nausea arose approximately one month at the end of a sequence of dose escalation from 1 g/day through 3 g/day for one month and finally 4 g/day for one month (Lawrence, 1993). A rapid reversal of the symptoms was found at 3 weeks after discontinuation of the nicotinic acid therapy. Rader et al (1992) reviewed the available cases of hepatotoxicity and side-effects from conventional and sustained-release nicotinic acid and concluded that adverse effects were frequently seen shortly after an abrupt change from unmodified to sustained-release preparations. Their paper summarised both the dose and the duration of therapy in the different cases of hepatic toxicity and showed that in general toxicity was associated with doses of 3 g/day or more, although there were 2 cases who took less than 1 g/day for short periods (0. A comparison of an immediate release formulation and a sustained-release formulation of nicotinic acid in two groups of 23 patients with low density lipoproteinaemia studied the sequential effect of 0. The therapeutic efficacy was similar for the two formulations but there were interesting differences in the side-effect profiles. About 39% of subjects on the immediate release formulation withdrew before completing the 3 g/day dose due to vasodilatory symptoms and fatigue, whereas 78% of subjects in the sustained-release group withdrew before completion of the study, primarily due to gastrointestinal tract symptoms, fatigue and changes in serum aminotransferases, indicative of hepatic dysfunction. The study of McKenney et al has been criticised because the dosage regimen of twice daily administration was considered to minimise the tolerability of the protocol and give the greatest potential for side-effects. The authors of the critique (Kennan et al, 1994) report that there was only a 5% drop-out rate as a result of intolerance and toxicity after one year in a study of 1119 subjects receiving 3 g (1 g three times a day) of immediate release nicotinic acid. The study of McKenney et al was also criticised because of the high top dose administered since drop-out rates of only 3-4% had been reported in studies where the maximum dose was 2 g/day. The average dose given at the end of the study was 2 g/day with a range from 1-3 g, which indicates the poor tolerability of doses greater than 2 g/day. A total of 9 patients showed elevated transaminase levels of at least 2 times the upper limit of normal. However 5 of these patients were on a combination therapy including nicotinic acid plus nystatin or a bile acid sequestrant. In 5 of the cases the transaminase elevation resolved while treatment with nicotinic acid continued and without a reduction in dose. Therefore this study demonstrates only mild hepatotoxicity in a group of subjects given controlled doses of sustained release nicotinic acid. Dalton and Berry (1992) describe a single case of a woman who presented with hepatotoxicity after taking crystalline nicotinic acid for a period of 2 years and sustained-release formulation for a period of only 2 days prior to admission. Her symptoms on admission to hospital included hypothermia, hypotension and metabolic acidosis, and the authors suggested that this may have been a result of the change from conventional to sustained-release nicotinic acid associated with prolonged flushing and possibly significant transcutaneous heat loss. This observation is ironic, since the sustained release formulation was primarily developed to minimise the skin flushing reaction associated with conventional nicotinic acid (Rader et al, 1992). Some studies have suggested that sustained-release formulations of nicotinic acid produce a greater incidence of hepatotoxicity (Christensen et al, 1961; Knopp, 1989; Mullin et al, 1989; Henkin et al, 1990), although this is not a consistent observation in all studies (Gibbons et al, 1995). Gray et al (1994) reported that the daily intakes of nicotinic acid in 42 elderly diabetic patients who developed hepatic dysfunction (2. Effects on prothrombin time have been reported in patients taking sufficient nicotinic acid to cause hepatic toxicity. Elevated prothrombin times have been reported in a small number of cases, which were associated with only mild elevation of transaminase levels so that blood-clotting disorders may become the limiting sign of hepatotoxicity in some cases. In contrast to the studies that have reported abnormal liver function in patients treated with nicotinic acid, a small study in the group of 30 patients with hyperlipidaemia who were given slow release nicotinic acid at 1 g/day for 2 months and then 2-3 g/day for 10 months reported a low incidence of symptoms other than skin flushing (which had an incidence of 26. There was no evidence of hepatic abnormalities as indicated by changes in serum aminotransferases, alkaline phosphatase or antipyrine test results (Chojnowska-Jezierska and Adamska-Dyniewska, 1998). A large number of studies have defined the efficacy and tolerability of both conventional and sustained or controlled-release nicotinic acid in the treatment of hypercholesterolaemia and hyperlipidaemias. The data from these studies provide adequate evidence of the hazard identification and some evidence of dose-response characterisation. A major problem with the use of such data for establishing an upper level is that the doses investigated were restricted to those that showed clinical efficacy in the conditions being treated (mostly 3 g/day), and there are few data available at lower levels (Rader et al, 1992). Hodis (1990) reported a case of acute hepatic failure, which was ascribed to treatment with 500 mg per day nicotinic acid, however there was no repeat challenge or other data to support causation (other than an absence of other recognised reasons). Glucose intolerance Although hyperglycaemia is a relatively rare side-effect associated with high doses of nicotinic acid, it can be of clinical significance. Administration of 3 g of nicotinic acid per day for 10-14 days to volunteers resulted in an increase in fasting blood glucose and immuno-reactive insulin in serum (Miettinen et al, 1969). An increase in blood glucose concentrations, glycosuria, elevated serum ketone bodies, and an increase requirement for hypoglycaemic medication were reported in 6 patients with diabetes mellitus, who were receiving between 1 g and 3 g of nicotinic acid daily for a period of 2 weeks or more (Molnar europa. Gray et al (1994) reported a high incidence of hyperglycaemia in elderly hyperlipidaemic patients who had been treated with high doses of nicotinic acid (average dose 1. Schwartz (1993) described a patient who was hospitalised with severe hyperglycaemia following treatment with 3 g of nicotinic acid per day for 4 months; administration of insulin and oral hypoglycaemics reversed and stabilised the blood glucose levels. Other effects and overall dose-response relationships Thrombocytopaenia, which resolved on cessation of nicotinic acid treatment, was reported in a single patient who developed hepatitis 10 years after the initiation of nicotinic acid treatment (Reimund and Ramos, 1994). The plasma concentrations of homocysteine were increased by 55% in patients with peripheral arterial disease who were treated with nicotinic acid (Garg et al, 1999). The 52 patients were a subgroup from a multicentre study in which patients were given increasing doses of 100, 500 and 1000 mg per day over periods of 3-4 weeks (in order to identify patients who tolerated nicotinic acid), following which the subjects were randomised to receive either placebo or nicotinic acid (up to 3 g per day). The plasma concentrations of homocysteine were measured at baseline, at randomisation and at 18 and 48 weeks after randomisation. After randomisation the levels increased further in those receiving nicotinic acid (to about 20 M at 18 and 48 weeks; n=25 and 24, respectively), but decreased in those on placebo (to about 12 M at 18 and 48 weeks; n=21 and 22, respectively). The clinical significance of this is unclear, but elevated plasma homocysteine is a recognised risk factor for coronary artery disease. Severe reversible cystoid macular oedema was reported in 3 patients receiving high-doses of nicotinic acid (Gass, 1973). A survey of 116 patients who had received nicotinic acid (3 g or more per day) for treatment of hyperlipidaemia and a similar number of patients who were not treated with nicotinic acid revealed an increased incidence of decreased vision associated with sicca syndromes, eyelid oedema or macular oedema (Fraunfelder et al, 1995). Because the majority of the data arise from studies designed to investigate the hypolipidaemic action of nicotinic acid, most of the data relate to doses of 1 g/day or more. In consequence, there are few data available on the tolerability and toxicity of doses less than 500 mg/day. In general the main adverse effect reported at intakes below the 500 mg/day has been flushing which is generally self-limiting in relation to continuation of treatment or intake of nicotinic acid.
We believe that the aggregate provisions recorded claims against generic companies seeking to limit the patent for the above matters are adequate based upon currently protection of Sanofi products) antifungal lock therapy discount diflucan 400mg with amex, competition law and trade available information antifungal nail pills order 200 mg diflucan otc. However antifungal pills diflucan 400mg line, given the inherent uncertainties practices fungus under toenail cure generic diflucan 400 mg otc, commercial claims fungus gnats effects on plants buy diflucan now, employment and wrongful related to these cases and involved in estimating contingent discharge claims antifungal horse cheap diflucan 100mg otc, tax assessment claims, waste disposal and liabilities, we could in the future incur judgments that could have pollution claims, and claims under warranties or indemnification a material adverse effect on our net income in any particular arrangements relating to business divestitures. They include: Most of the issues raised by these claims are highly complex and Provisions for product liability risks, litigation and other amount subject to substantial uncertainties; therefore, the probability of to 1,288 million in 2018. These provisions are mainly related loss and an estimation of damages are difficult to ascertain. In either case, a brief description of the Provisions for environmental risks and remediation amount to nature of the contingent liability is disclosed and, where 680 million in 2018, the majority of which are related to practicable, an estimate of its financial effect, an indication of the contingencies that have arisen from business divestitures. In the cases that have been settled or adjudicated, or where Sanofi Pasteur Hepatitis B Vaccine Product Litigation quantifiable fines and penalties have been assessed, we have Since 1996, more than 180 lawsuits have been filed in various indicated our losses or the amount of provision accrued that is French civil courts against Sanofi Pasteur and/or Sanofi Pasteur the estimate of the probable loss. In March Depakine Product Litigation in France 2012, Sanofi Pasteur and its former pharmacist in charge. In March 2016, the investigating judges decided claims have been filed against a French affiliate of Sanofi seeking to dismiss the proceedings. Several civil parties appealed against indemnification under French law for personal injuries allegedly this decision. On June 4, 2018, the Prosecutor General sustained by children in connection with the use of sodium requested confirmation of the dismissal. The case has been valproate by their mothers during pregnancy to treat their adjourned for deliberation on June 14, 2019. In October 2017, the French Supreme Court (Cour de cassation) these actions are held in several jurisdictions in France. French affiliate filed a motion to the French Supreme Court to In January 2018, the Appeal Court of Bordeaux found a causal reverse the decision rendered by the Court of Appeal of Orleans link between hepatitis B vaccine and multiple sclerosis. Sanofi (France) against Sanofi in November 2017 ordering payment of Pasteur Europe appealed this decision before the French approximately 2 million to the plaintiff and 1 million to the Supreme Court (Cour de cassation). District Court for the District of New Medicaux) and the healthcare professionals, in October 2018, the Jersey. The actions are held in several the French government has, through the 2017 Finance law jurisdictions, including the federal and/or state courts of adopted on December 29, 2016, set up a public fund which is Louisiana, New Jersey, California, Delaware and Illinois. The meant to compensate loss or injury actually suffered in relation to Eastern District of Louisiana Federal Court in New Orleans has the prescription of sodium valproate and its derivatives. The fund entered a scheduling order setting the first bellwether trial for entered into force on June 1, 2017. It is not possible, at this stage, to assess reliably raised issue of conflict of interest of certain appointed experts, the outcome of these lawsuits or the potential financial impact on which led to those experts being either removed or replaced as the Company. It is not possible, at this stage, to assess It is not possible, at this stage, to assess reliably the outcome of reliably the outcome of this lawsuit or the potential financial these cases or the potential financial impact on the Company. Sanofi and Regeneron initially asserted, patent infringement actions against all those companies and on among other defenses, invalidity and non-infringement defenses. June 29, 2009, the Federal Court of Canada ruled that the patent In January 2016, Sanofi and Regeneron informed the District asserted by Sanofi was invalid. The Riva Section 8 case, which had been stayed pending resolution of the Supreme Court of the validity judgment and injunction ruling in the Federal Appeal, was settled following court-sponsored mediation in Circuit. Amgen filed a petition for rehearing by the full Federal Ontario Superior Court of Justice asserting damages under the Circuit in December 2017 which was denied. The Ontario Action the District Court has set a jury trial on invalidity to begin in was stayed pending exhaustion of appeals in the Section 8 February 2019, with a jury trial on damages and possibly willful damages action and, despite having received full compensation infringement immediately to follow, should Sanofi and Regeneron in the Section 8 action, was reinitiated by Apotex after the lose on validity. The District Court also allowed legal principles applied in the ramipril invalidity decision were each side to file one summary judgment motion, both of which unsound and in the fall of 2018 Sanofi sought to amend its were denied in January 2019. The trial for this matter, originally expected for fall Amgen has filed three separate patent infringement lawsuits 2019, will now likely be delayed significantly. The claims of this relief and unspecified damages; Sanofi has counterclaimed patent relate to , among other things, human monoclonal invalidity. New oral on the basis that, inter alia, the claims are invalid for prohibited proceedings are scheduled for April 2019. The validity of these two Japanese patents was separately In April 2017, Immunex filed a complaint in the U. The Spirit proceeding was declaratory judgment that it is entitled to , among other things, consolidated with the Apotex proceeding. The security bond posted by Sanofi in connection with the counsel for the investigation and prosecution of the claims in the preliminary injunction obtained in 2007 was subsequently case. In light of the Apotex settlement, the and records, as well as its request for an audit. On March 24, Commonwealth has requested that the Court consider a set of 2017, the Trustee sought leave to amend its complaint for a legal issues separate from trial that could simplify the trial. In second time to assert a breach of contract claim with respect to December 2015, the Court held that the relevant statute does not the Production Milestone, which request was granted on preclude the Commonwealth from seeking damages in cases August 23, 2017. On October 6, 2017, the Court on the issue of the invalidity of the patent was denied in Trustee filed a motion for summary judgment with respect to its November 2015. A number of other outstanding claims has indemnification obligations that generally expired on remain unresolved. Bayer is seeking indemnification for 2019, subject to an extension for claims related to certain types damages allegedly suffered in several hundred individual of product liability notified before such date. Aventis Animal Nutrition Retained Liabilities Aventis CropScience Retained Liabilities Aventis Animal Nutrition S. The representations and warranties and the indemnification are indemnification undertakings are subject to an overall cap of subject to a cap of 836 million, except for certain legal 223 million, with a lower cap for certain environmental claims. There are various periods of limitation depending upon the nature or subject of the the demerger of the specialty chemicals business from Hoechst indemnification claim. Under the demerger agreement between Hoechst and Celanese, Hoechst expressly excluded Since December 2005, Aventis Agriculture and Hoechst GmbH any representations and warranties regarding the shares and have concluded several settlement agreements to resolve a assets demerged to Celanese. Thereafter, Celanese remains liable for known from certain locally concentrated pollutions in the sites taken over environmental claims specified in 2013. In January 2004, two minority shareholders of Rhodia and their Infraserv Hochst Retained Liabilities respective investment vehicles filed two claims before the Commercial Court of Paris (Tribunal de Commerce de Paris) By the Asset Contribution Agreement dated December 19/20, 1996, against Aventis, to which Sanofi is successor in interest, together as amended in 1997, Hoechst contributed all lands, buildings, and with other defendants including former directors and statutory related assets of the Hoechst site at Frankfurt Hochst to Infraserv auditors of Rhodia from the time of the alleged events. Infraserv Hochst undertook to indemnify claimants seek a judgment holding the defendants collectively Hoechst against environmental liabilities at the Hochst site and with liable for alleged management errors and for alleged publication respect to certain landfills. These shareholders seek a finding of joint reimburse current and future Infraserv Hochst environmental and several liability for damages to be awarded to Rhodia in an expenses up to 143 million. As a former operator of the land and amount of 925 million for alleged harm to it (a derivative action), as a former user of the landfills, Hoechst may ultimately be liable for as well as personal claims of 4. In 2006, the Commercial Court of Paris related to liabilities arising before Closing. Sanofi is working to investigate the validity of decision before the French Supreme Court (Cour de cassation). Provisions for discounts, rebates and sales returns Adjustments between gross sales and net sales, as described in Note B. Personnel costs Total personnel costs include the following items: ( million) 2018 2017(a) 2016(a) Salaries 6,547 6,592 6,424 Social security charges (including defined-contribution pension plans) 1,954 1,977 1,948 Stock options and other share-based payment expense 282 258 250 Defined-benefit pension plans 261 275 273 Other employee benefits 225 219 224 Total 9,269 9,321 9,119 (a) Excluding personnel costs for the Animal Health business: immaterial in 2017 and 0. The total number of registered employees (excluding those of the compared with 106,566 as of December 31, 2017 and 106,859 Animal Health business) was 104,226 as of December 31, 2018, as of December 31, 2016. Other operating income In 2018, this line item includes 225 million of expenses relating to the agreement with Regeneron, versus 11 million in 2017 and Other operating income totaled 484 million in 2018, versus 10 million in 2016. In 2017, Sanofi recognized an impairment mature products in Latin America and some Consumer loss of 87 million against property, plant and equipment Healthcare products in Europe (326 million in 2018, 90 million associated with the dengue vaccine project. Other operating expenses totaled 548 million in 2018, compared with 233 million in 2017 and 482 million in 2016. Restructuring costs and similar items Restructuring costs and similar items amounted to 1,480 million in 2018, 731 million in 2017 and 879 million in 2016, and comprise the following items: ( million) 2018 2017 2016 Employee-related expenses 517 336 650 Expenses related to property, plant and equipment and to inventories 162 221 139 Compensation for early termination of contracts (other than contracts of employment) 352 61 31 Decontamination costs 5 (4) 3 Other restructuring costs 444 117 56 Total 1,480 731 879 Restructuring costs recognized in 2018 included: (b)a provision of 283 million booked as of December 31, 2018 for penalties arising from the restructuring of the (a)termination benefit payments of 517 million in 2018, immuno-oncology research and development agreement including provisions associated with the headcount with Regeneron, and in particular on termination of the adjustments in Europe announced in December 2018. Other gains and losses, and litigation (d)the costs of transferring the infectious diseases early stage In 2018, the line item Other gains and losses, and litigation R&D pipeline and research unit. Those transfer costs consists of the pre-tax gain of 502 million arising on the amounted to 252 million and primarily consist of payments divestment of the European Generics business (completed to Evotec over a five-year period, including an upfront September 30, 2018), net of separation costs (see Note D. Consequently, Sanofi recognized an impairment loss reflecting the difference between the historical acquisition cost of its shares in Alnylam and their market value. In 2018, 2017 and 2016, the impact of the ineffective portion of hedging relationships was not material. Income tax expense Sanofi has elected for tax consolidations in a number of countries, principally France, Germany, the United Kingdom and the United States. The difference between the effective tax rate and the standard corporate income tax rate applicable in France is explained as follows: (as a percentage) 2018 2017 2016(a) Standard tax rate applicable in France 34. In 2016, entities subject to corporate income tax in France were liable to pay an additional tax contribution in respect of amounts distributed by the entity. In determining the amount of the deferred tax liability for 2018, 2017 and 2016, Sanofi took into account changes in the ownership structure of certain subsidiaries. For 2016, it includes the effects of changes in tax rates in various countries, particularly in France, Hungary, Italy, Japan and the United States. For the periods presented, the amount of deferred tax assets recognized in profit or loss that were initially subject to impairment losses on a business combination is immaterial. Transactions between those companies, and between the parent company and its subsidiaries, are eliminated the principal related parties are companies over which Sanofi when preparing the consolidated financial statements. Transactions with companies over which Sanofi has significant influence, and with joint ventures, are presented in Note D. Sanofi has not entered into any material transactions with any key management personnel. The table below shows the aggregate top-up pension obligation lump-sum retirement benefits payable to key management in favor of certain corporate officers and Executive Committee personnel: members, and the aggregate amount of termination benefits and ( million) 2018 2017 2016 Aggregate top-up pension obligation 59 68 72 Aggregate termination benefits and lump-sum retirement benefits 10 9 8 D. Disclosures about major customers and customers in order to set credit limits and risk levels and asking credit risk for guarantees or insurance where necessary, performing controls, and monitoring qualitative and quantitative indicators of Credit risk is the risk that customers (wholesalers, distributors, accounts receivable balances such as the period of credit taken pharmacies, hospitals, clinics or government agencies) may fail and overdue payments. The Vaccines segment comprises, for all geographical territories (including certain territories previously included in the Sanofi D. This segment also includes associates whose activities are related to pharmaceuticals, in particular our share of Regeneron. Other segment information the principal investments accounted for using the equity method are: for the Pharmaceuticals segment, Regeneron the tables below show the split by operating segment of (i) the Pharmaceuticals, Inc. Acquisitions of intangible assets and property, plant and equipment correspond to acquisitions paid for during the period. Information by geographical region the geographical information on net sales provided below is exclude financial instruments, deferred tax assets, and based on the geographical location of the customer. An analysis of those assets were classified as of December 31, 2016 in the line items line items is set forth below: Assets held for sale or exchange and Liabilities related to 2016 Assets Property, plant and equipment 811 Goodwill 1,560 Other intangible assets 2,227 Investments accounted for using the equity method 12 Other non-current assets 41 Deferred tax assets 180 Inventories 629 Accounts receivable 471 Other current assets 83 Cash and cash equivalents 362 Total assets held for sale or exchange 6,376 Liabilities Long-term debt 6 Non-current provisions 134 Deferred tax liabilities 198 Current debt 148 Accounts payable 241 Other current liabilities 438 Total liabilities related to assets held for sale or exchange 1,165 As of December 31, 2016, short-term debt owed by Animal policies described in Note B. In accordance with the accounting to these assets and liabilities are not included in the table above.
Although studies involving high Acanthamoeba and Balamuthia infections and which can doses of praziquantel in pregnant animals have failed lead to relapse following apparently effective treatment antifungal drinks order diflucan no prescription. The use of praziquantel in pregnant nonopportunistic infection of the cornea anti fungal oil for nails 100 mg diflucan, which responds women and in children younger than 4 years has not been well to treatment with chlorhexidine gluconate and poly well studied antifungal washing detergent discount 400mg diflucan free shipping, and both the risks and the benefits must be hexamethylene biguanide in combination with propamidine considered antifungal antibodies purchase diflucan pills in toronto. Praziquantel does appear in human milk antifungal oral gel quality 100 mg diflucan, and isethionate (Brolene) fungus gnats uc davis buy cheap diflucan 150 mg line, hexamidine (Desomodine), or neomy mothers should not breast-feed during treatment and for cin. In a large series of Acanthamoeba keratitis patients with 72 h following treatment. Concomitant administration of a positive microbiologic diagnosis at presentation, nearly rifampin should be avoided. Praziquantel causes ultrastructural changes in the hel minth tegument, causing increased permeability to cal Praziquantel (Biltricide) (Bayer) cium ions. However, the patient may Praziquantel is supplied as 150-, 500-, and 600-mg not notice them since they are sometimes completely de scored tablets; is a pyrazinoisoquinoline derivative; and stroyed in the intestine. Effective medications It is an antimalarial agent especially effective against include topical polyhexamethylene biguanide, propamidine Plasmodium vivax exoerythrocytic forms, terminating isethionate (Brolene), chlorhexidine digluconate 0. Cautious introduction of topical Tablets have a bitter taste and may be crushed and added steroids along with antiamebic therapy helped resolve the to a sweet liquid or fruit to make them more palatable. Toxicity Patients may experience abdominal pain, cramps, or Pyrantel Pamoate (Antiminth) (Pfizer) epigastric distress. Granulocytopenia and granulocy Preparation tosis, hypertension, and arrhythmia are rare. However, it is associated with more side effects the drug should not be used in patients with depressed and is being replaced with the benzimidazoles, which are blood counts resulting from other illnesses or concurrently more effective. Because pri Administration maquine phosphate can cause hemolytic anemia, patients Pyrantel pamoate may be taken with food, milk, or juice. Toxicity Administration of other bone marrow depressants as he Reactions are not frequently encountered, but when they molytic agents should be avoided. The drug should not be do occur, they involve primarily the gastrointestinal sys prescribed during pregnancy. They include anorexia, nausea, vomiting, abdominal pain or cramps, diarrhea and tenesmus, and transient elevations of the transaminase enzyme levels. Propamidine Isethionate (Brolene) (Aventis, Canada) Contraindications Studies of pregnant animals have failed to show any harm Preparation to the fetus, but there is no experience with pregnant hu Brolene is provided as a 0. There are few published data on the use of the drug infective agent that is used to treat keratitis caused by in children younger than 2 years. Brolene is administered topically each hour for the first few days and every few hours thereafter for a minimum Comment course of 1 month. The drug is a neuromuscular blocking agent, resulting in spastic paralysis of the adult worms. Excellent results are Toxicity obtained when treating patients with ascariasis, hook the drug may cause keratopathy; this condition is revers worm, and pinworm infections. While there is some activ ible but may be confused with persistent or recurrent ity against Trichostrongylus, the drug is not active against infection. Treatment of Parasitic Infections 753 Pyrethrin with Piperonyl Butoxide (Rid) insomnia. For patients with toxoplasmosis being treated (Pfizer, Others) with high doses, periodic complete blood counts, includ ing platelet counts, are recommended. Preparation Hypersensitivity reactions, occasionally severe (Stevens Rid is an insecticide mixture of permethrin and piperonyl Johnson syndrome, toxic epidermal necrolysis, erythema butoxide, which is used as a synergist and also to treat multiforme, and anaphylaxis), can occur, particularly human louse infestations. Administration Rid is applied topically as a shampoo to the affected area Contraindications after wetting. After application, it should be left for 10 min the drug should not be used in pregnancy, and determi and then rinsed thoroughly. Hair should be combed with a nation of human chorionic gonadotropin levels is recom fine-tooth comb to remove dead lice and nits. Caution is recommended for patients with seizure disor Toxicity ders and impaired hepatic or renal function. Daraprim is Rid may cause allergic reactions in individuals allergic to also contraindicated for patients with documented mega ragweed. Contraindications Comment the drug should not be used near eyes or mucous mem Pyrimethamine acts against the asexual erythrocytic stage branes. Preparation Therefore, the drug is often combined with a sulfonamide Daraprim is supplied as 25-mg tablets; it is a potent or sulfone, which provides sequential, synergistic inhibi folic acid antagonist used as an antimalarial agent and tion of the folate biosynthesis pathway. Administration Preparation the drug may be pulverized and mixed with sweet liquid Quinidine gluconate is supplied as a solution containing or fruit juice. Concurrent administration of folinic acid 80 mg/ml for injection and is used to treat severe malaria. Daraprim is indicated for the treatment of toxoplasmosis when used Administration conjointly with a sulfonamide, providing synergy with this the drug is given i. Although Daraprim is used to treat mg is infused in 40 ml of 5% glucose at a rate of 1 ml/min. It is also used for chemoprophylaxis of malaria; however, Toxicity there is widespread resistance throughout the world. Quinidine causes gastrointestinal irritation, with nausea, vomiting, and diarrhea. Toxicity Other side effects that have been reported include blurred Gastrointestinal complaints (anorexia, nausea, vomit vision, headache, belly pain, and ringing in the ears and ing, diarrhea, and abdominal pain and tenderness) are temporary loss of hearing. Occasional elevation of the glutamic terval as indicated by an electrocardiogram, ventricular oxaloacetic transaminase level in serum has been reported, arrhythmia, hypotension, and hypoglycemia can result as have skin rashes, headache, dizziness, drowsiness, and from the use of this drug at treatment doses. Use should be avoided for patients with myasthenia gravis, acute infections with Administration fever, previous hypersensitivity to quinidine, and cardiac Spiramycin is given orally on an empty stomach or by i. Toxicity Quinine Sulfate or Quinine Dihydrochloride Rovamycine can cause gastrointestinal disturbances and (Many Manufacturers) occasional allergic reactions. More rare side effects have included bloody stools, chest pain, fever, heartburn, irreg Preparation ular heartbeat, nausea, recurrent fainting, stomach pain Quinine sulfate is supplied as 3-grain (186-mg) or 6-grain and tenderness, vomiting, and yellow eyes or skin. Quinine dihydrochloride comes in 2-ml ampoules containing 300 mg/ml (not Contraindications available in the United States). It is used to treat malaria the drug should not be given to patients with impaired and is important in areas where i. More severe reactions are related to inability to excrete the Administration drug normally and to high doses. Pneumonitis, arthralgias, myalgias, bradycardia, electrocardiogram changes (T-wave depres Contraindications sions, increased O-T interval, and fusion of the S-T segment Rare reactions include renal failure, acute hemolysis, hy and T wave), abnormal liver function, headache, and mild poprothrombinemia, purpura, agranulocytosis, asthma, rash also occur. After one dose, an idiosyn lytic anemia, thrombocytopenia, and anaphylaxis associated cratic hypersensitivity reaction consisting of flushing, with urticaria, laryngeal edema, and visceral collagens. Massive hemolysis Contraindications and hemoglobinuria occasionally occur during the treat the drug should not be used in patients with myocardi ment of falciparum malaria. Quinine may aggravate the tis, hepatitis, liver disease, or concurrent bacterial and symptoms of myasthenia gravis. Treatment should be stopped if there is progressive proteinuria, severe arthralgias, or rash. More severe reactions may include leukopenia, agranulocytosis, Spiramycin (Rovamycine) (Aventis) and electrocardiographic changes. Preparation Comment Rovamycine is supplied as a tablet or in solution and is Pentostam (sodium stibogluconate; sodium antimony a macrolide antibiotic similar to leucomycin. This drug gluconate) is a pentavalent antimony compound that is Treatment of Parasitic Infections 755 used to treat all forms of leishmaniasis (cutaneous, mu Thiabendazole (Mintezol) (Merck) cocutaneous, and visceral). This drug is a well-established antileishmanial agent that has been in use for some time. Preparation It is thought to inhibit glycolysis enzymes within the or Mintezol is supplied as a flavored suspension containing 125 ganisms. Administration Less frequently, diarrhea, epigastric pain, pruritus, drowsi ness, lethargy, and headache occur. Side effects include A 10% solution must be prepared and used within 30 min Stevens-Johnson syndrome, tinnitus, visual disturbance, of preparation as a slow i. Treatment may cause migration of Immediate nausea, vomiting, loss of consciousness, and Ascaris organisms to the esophagus, mouth, and nose. Proteinuria is Contraindications commonly seen during therapy, but it is not a parameter Patients with known hypersensitivity or with renal or used to monitor therapy unless other renal function stud hepatic impairment should not use thiabendazole. Fever is also common but is usually low causes dizziness and drowsiness and should not be used grade. It is Blepharitis, conjunctivitis, photophobia, and excessive also contraindicated as prophylaxis for pinworm infections; lacrimation may occur during treatment. Kidney failure, susceptible worm infections should be confirmed by labora blood dyscrasias (including pancytopenia), shock, and tory testing prior to drug use. The drug should be administered under close medical Comment supervision and should not be used in pregnant women Thiabendazole blocks glucose uptake, depletes glucose or in patients with hepatic or renal insufficiency. Suramin was introduced in 1920 for the treatment of African trypanosomiasis (sleeping sickness). It is consid Tinidazole (Tindamax) ered the drug of choice for treatment of early African try (Mission Pharmaceuticals) panosomiasis with Trypanosoma brucei rhodesiense when the central nervous system is not involved. Pentamidine is thought to be more effective than suramin for treating Preparation early African trypanosomiasis caused by T. The mechanism of action of suramin is thought to 1-g tablets or as an oral suspension of 200 mg/ml. Interruption of breast-feeding of trichomoniasis caused by Trichomonas vaginalis in is recommended during tinidazole therapy and for 3 days both female and male patients. Because trichomoniasis is a sexually transmitted disease with Comment potentially serious sequelae, partners of infected patients the nitro group of tinidazole is reduced by cell extracts should be treated simultaneously in order to prevent of Trichomonas. Tindamax oral tablets are indicated for the result of this reduction may be responsible for the anti treatment of giardiasis caused by Giardia lamblia in both protozoal activity. The mechanism by which tinidazole adults and pediatric patients older than 3 years. Tindamax exhibits activity against Giardia and Entamoeba species oral tablets are indicated for the treatment of intestinal is not known. It is not indicated in the treatment of asymptom significance of such an effect is not known. Tinidazole, like metronidazole, may interfere with certain types of determinations of serum chemistry values, Administration such as aspartate aminotransferase, alanine aminotrans Tinidazole is given orally with or after meals. After oral ferase, lactate dehydrogenase, triglyceride, and hexoki administration, it is rapidly and completely absorbed. Alcoholic beverages should be avoided while taking Tin damax and for 3 days afterward. Triclabendazole (Egaten) (Novartis) Toxicity Tinidazole can cause nausea, vomiting, metallic taste, Preparation and rash. Convulsive seizures and peripheral neuropa Triclabendazole is used in veterinary practice and is thy, the latter characterized mainly by numbness or par also being used to treat human infections with liver esthesia of an extremity, have been reported to occur in and lung flukes. The drug is not available in the United patients treated with nitroimidazole drugs including tini States. The appearance of abnormal neurologic signs demands the prompt discontinuation of Administration Tindamax therapy. Tinidazole should be administered Egaten is given orally after a meal as a single or split dose with caution to patients with central nervous system of 10 mg/kg. A dose of 20 mg/kg is safe and effective for diseases or those with evidence of or history of blood patients with acute fascioliasis when a single dose has dyscrasia. Contraindications Toxicity As with other nitroimidazole derivatives, tinidazole may Triclabendazole can cause abdominal pain; however, most enhance the effect of warfarin and other coumarin antico studies reported no side effects. The tolerance of triclaben agulants, resulting in a prolongation of the prothrombin dazole is considered excellent. Alcoholic beverages and preparations containing episodes of upper abdominal pain and slight fever and ethanol or propylene glycol should be avoided during some mild and limited disturbances in liver function. These tinidazole therapy and for 3 days afterward because effects may be due to the paralysis and/or death of the abdominal cramps, nausea, vomiting, headaches, and flukes, resulting in the release of antigens or toxic products flushing may occur. Similar findings in alcoholic patients using metronidazole and disulfiram have been reported in various preliminary studies: tricla concurrently. Because animal reproduction studies are bendazole has been reported to decrease parasite motility, not always predictive of the human response and because but the exact mode of action of this drug is unknown. Some there is some evidence of mutagenic potential, the use of patients may require a second course of therapy. Tinidazole is excreted Use of this drug may cause secondary cholangitis due to in breast milk in concentrations similar to those seen in worm destruction. It can be detected in breast milk for up to 72 h be nonmutagenic and nonteratogenic. Multiple synergistic genes using polymerase chain reaction and enzyme-linked interactions between atovaquone and antifolates against immunosorbent assay-based technology. Modern malaria gonimiasis with triclabendazole: clinical tolerance and drug chemoprophylaxis. Fluorescent Leishmania: cases of fascioliasis: clinical and microbiological features. The Pharmaceutical Press, London, United the Sanford Guide to Antimicrobial Therapy, 33rd ed. When a labora Specimen type, specimen stability, tory selects its collection methods, the decision should be based on a thorough and need for preservation understanding of the value and limitations of each. One of the most important aspects of specimen collection is that the final laboratory results based on para Preservation of specimens site recovery and identification will depend on the initial fixation of the organ Preservatives isms (5, 9, 17, 31, 33). As a Quality control for stool fixatives part of any overall total quality management or continuous quality improve Procedure notes for use of preservatives ment program for the laboratory, the generation of test results must begin with Procedure limitations for use of preservatives stringent criteria for specimen acceptance or rejection.
Crucially quince fungus buy discount diflucan 150mg online, most clinicians working in resource-poor areas will be Surgeons should be involved at an early stage if surgical drainage unable to measure ScvO2 and implement this strategy of treatment fungus gnats temperature order genuine diflucan online. This is infammation of the lungs with increased possible fungus mold 50mg diflucan with visa, after taking blood cultures bracket fungus definition order diflucan 150 mg on-line. Giving efective antibiotics within vascular permeability characterised by bilateral infltrates on chest the frst hour has been associated with increased survival in septic X-ray fungus roots generic 400 mg diflucan with visa, not caused by cardiac failure fungus around nose order diflucan 50mg with amex. The study showed that a protocol of goal-directed therapy during the frst 6 hours of admission, aimed at achieving a balance between oxygen delivery and oxygen demand, reduced hospital mortality from 46% in the control group to 30% in the experimental group. Volume resuscitation alone was sufcient to correct ScvO2 in 36%, transfusion in an additional 50% and inotropes in 13. We can conclude that this protocol, applied early with frequent review, to patients with severe sepsis can reduce mortality. ScvO2 is probably a useful resuscitation goal, however it is not possible to say exactly which aspects of this protocol were most benefcial. This was a small, single-centre, unblinded study with a high control group mortality. Two meta-analyses have shown reduction in mortality, but only in studies of low dose, long duration steroid therapy. The targets of pressure hydrocortisone to placebo in septic shock, showed faster shock reversal <30cmH O and tidal volume 6ml. This is limited if the patient has a the 2008 Surviving Sepsis Guidelines suggest giving low dose metabolic acidosis (pH <7. Tere is no shown a mortality beneft, but did improve secondary endpoints such diference in the efcacy of jejunal versus gastric feeding, but they as oxygenation, duration of ventilation and use of rescue therapies. Gastric emptying is frequently the rate-determining step so, where available, motility agents such as erythromycin and Semi-recumbent positioning metoclopramide may be helpful in patients with feed intolerance and Nursing ventilated patients in the semi-recumbent position (45 high gastric residual volumes. If available and afordable, parenteral degrees, head up) has been shown to reduce the incidence of 30 nutrition may be considered in patients who cannot be fed sufcient ventilator-associated pneumonia. Non-invasive ventilation, subglottic drainage and support in Canadian intensive care units was associated with more days use of heat and moisture exchange flters, instead of heated water 42 of enteral nutrition and improved clinical outcomes. Ventilatory weaning protocols A protocol for weaning patients from mechanical ventilation is Laparotomy and peritonitis is not a contraindication to enteral feeding helpful. Once a patient is improving and meets certain criteria, daily and several studies have shown benefts of early nasojejunal feeding in these patients. The above studies used nasojejunal feed prepared in hospital T-piece or low level of pressure support) with a spontaneous awakening kitchens (and include the recipes). Patients are often given liquidized trial in which sedation (but not analgesia) is stopped, can reduce food, soup, milk etc. Steroids in sepsis The Surviving Sepsis Guidelines9 recommend stress ulcer prophylaxis Patients on long term steroid therapy or with known adrenocortical with ranitidine, but this can potentially increase the risk of ventilator insufciency require steroid replacement during critical illness. Use of muscle relaxants without blood glucose control adequate continuous sedation is unacceptable. Physical restraint may be preferable to chemical sedation in that intensive insulin therapy improved some markers of morbidity but some situations, but should be carefully and selectively employed. It not mortality, and 19% of patients in the treatment group developed should only be used if patients are not competent to make decisions, hypoglycaemia. It is Volume Substitution and Insulin Terapy in Severe Sepsis) was important to keep trying to communicate with the patient. They found a much lower incidence of severe hypoglycaemia Renal support and lower mortality in the conventional control group. The risk of renal failure can be reduced by early fuid resuscitation, maintaining renal Septic patients are at risk of both hypo and hyperglycaemia, whether perfusion pressure and cardiac output (with inotropes if necessary), or not they are treated with glucose and insulin. Tere is be checked in all sick patients, but close monitoring of blood glucose no evidence for using low dose dopamine for renal protection. Four to six-hourly acidosis should be treated by optimising the circulation, not with subcutaneous insulin, adjusted according to blood glucose, is an 9 sodium bicarbonate. Mental state may improve with resuscitation and provides unfractionated or low molecular weight heparin, unless contraindicated an important marker of organ perfusion. Benzodiazepines may cause compression stockings may also be used for very high risk patients or if heparin is not given. Where available, haloperidol is a useful drug in Sepsis bundles are clinical guidelines that combine therapies, aiming confused patients. Only early antibiotic use has been risk of prolonged muscle weakness (critical illness polyneuropathy), proven to be benefcial. Many hospitals in developing countries do not have the resources to implement sepsis guidelines and bundles. In developing countries this well resourced and all able to measure central venous pressure, arterial could include giving oxygen and recording basic observations, fuid blood gases and blood cultures. Compliance with bundle targets for resuscitation, and early administration of antibiotics after blood blood cultures, antibiotics and central venous pressure independently cultures have been taken. She is apyrexial with a heart rate of 130min-1 and a blood pressure of 140/95mmHg. She is seen by a junior surgeon who fnds a soft abdomen, diagnoses gastroenteritis and treats her with oral rehydration solution. Her respiratory rate is 35min-1, your saturation monitor is not picking up a signal. Further history from her mother reveals that her waters broke 2 days before delivery. Her delivery was uncomplicated, with no excessive bleeding and the placenta appeared intact. You suspect genital tract sepsis, so start amoxycillin 2g 6 hourly, metronidazole 500mg 8 hourly and gentamicin 5mg. You take vaginal swabs for gram stain and culture and send urine for culture as soon as possible. You perform a thorough physical examination, looking for other sources of sepsis and take a more complete history. You ask an obstetrician to confrm your diagnosis, do a pelvic ultrasound to look for retained products and to assess whether there is an indication for surgery. You send blood for full blood count, malaria screen, urea and electrolytes and check blood glucose. You would like a chest Xray to look for air under the diaphragm or signs of infection, but this is not available in the evenings. Gram stain of the vaginal swab shows gram positive cocci and gram negative bacilli. Low platelets occur in severe sepsis and may indicate disseminated intravascular coagulation. The haemoglobin is relatively high for a woman who has just had a baby, which may refect dehydration, consistent with the slightly raised sodium and urea. The low bicarbonate and raised anion gap suggest a metabolic acidosis, probably lactic acidosis. Gram stain shows mixed organisms for which she is on appropriate broad spectrum antibiotics. If culture shows group A streptococcal infection you could consider adding benzylpenicillin. Septic shock unresponsive to fuid: start vasopressor, continue goal-directed therapy. You start norepinephrine (epinephrine would be your second choice), 5mg in 500ml through a paediatric (60drops. You tell them to call you if these goals are not met, heart rate or respiratory rate increase, saturation or conscious level are reduced. You have a busy night: frequent fuid boluses are required for decreased urine output, reduced blood pressure or cool peripheries. Oxygen saturations drift down when she is sleeping, but they improve with sitting her up in bed and deep breathing. The next morning, after 5 litres of fuid, she is beginning to improve and the noradrenaline is gradually turned of. You ask for chest physiotherapy, encourage oral fuids and diet, and recheck blood tests. The diagnosis may not be obvious and patients may not always have a fever, but recognising when a patient is sick is vital. This may include teaching sessions for doctors and nurses, organisational change to allow more frequent observations and improve stafng levels, and resources such as sphygmomanometers and saturation monitors. However, early recognition and timely simple interventions are the key to survival. Limitations on resources make implementation of the national prospective multicenter study. Rapid increase in antibiotics, with frequent review to adjust treatment, can be achieved hospitalization and mortality rates for severe sepsis in the United in any hospital. Bundled care for septic shock: An analysis of clinical Medicine Consensus Conference: defnitions for sepsis and organ trials. Impact of time to antibiotics on survival in patients patients in hospital: recognition of and response to acute illness with severe sepsis or septic shock in whom early goal-directed in adults in hospital. London: Natioanl Institute for Health and Clinical therapy was initiated in the emergency department. Surviving Sepsis Campaign: International guidelines traditional tidal volumes for acute lung injury and acute respiratory for management of severe sepsis and septic shock. Higher versus Lower Positive End-Expiratory Pressures in for Fluid Resuscitation in the Intensive Care Unit. Positive End-Expiratory Pressure Setting in Adults With the Scandinavian Critical Care Trials Group). Ventilation Strategy Using Low Tidal Volumes, Recruitment Maneuvers, and High Positive End-Expiratory Pressure for 12. Occult hypoperfusion and mortality in patients with Acute Lung Injury and Acute Respiratory Distress Syndrome. Cochrane Database of Systematic Reviews 2006, Issue pneumonia in mechanically ventilated patients: a randomised trial. Pulmonary-artery ventilation and conventional mechanical ventilation in patients with versus central venous catheter to guide treatment of acute lung acute respiratory failure. Results of the Sepsis Occurrence in Acutely Ill Patients identifying patients capable of breathing spontaneously. Norepinephrine plus dobutamine versus epinephrine alonefor management of septic shock: a randomised trial. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. A Multicenter, Randomised, Controlled Clinical Trial of Transfusion Requirements in Critical Care. Available at: haemofltration on outcomes of acute renal failure: a prospective guidance. Early Enteral Feeding by Nasoenteric Tubes in Patients Continuous Renal-Replacement Therapy in Critically Ill Patients. Prophylaxis of Venous Thromboembolism; 2002: Nontraumatic Intestinal Perforation and Peritonitis. Intensive insulin therapy in the critically ill international guideline-based performance improvement program patients. Intensive insulin therapy in patient with implementing current sepsis guidelines in Mongolia. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. Precipitants of post-traumatic stress disorder following and Childbirth A guide for Midwives and Doctors, World Health intensive care: A hypothesis generating study of diversity in care. His injuries included rib fractures, a ruptured spleen and an unstable pelvic fracture. He was admitted to the intensive care unit following external fxation of his pelvis, laparotomy and splenectomy. Estimated blood loss was over 4 litres, which was replaced with a combination of blood, fresh frozen plasma and platelets. At Summary the end of the case the patient had a metabolic acidosis, a mild coagulopathy, mild anaemia and was Abdominal compartment hypothermic. However his ventilatory condition associated with requirements increased, with peak inspiratory pressure rising from 22 to 38cmH20 in order to maintain signifcant morbidity and acceptable tidal volumes. This article includes remained greater than -8 and his urine output remained less than 0. Following diagnosis and management discussion with the surgical team he was taken for decompressive laparotomy (laparostomy). No active bleeding was found but large intra abdominal and retro-peritoneal haematomas were identifed.
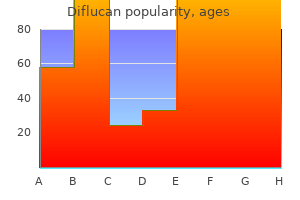
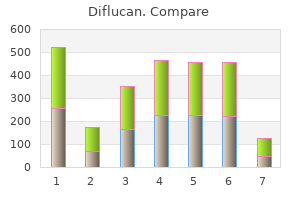
Buy diflucan with visa. Monocan 100 Antifungal Antibacterial Capsule 100 mg Review in Hindi.